Breast cancer is the most common type of cancer in the United Kingdom. Usually a lump or thickened breast tissue is the first noticeable symptom, although most breast lumps are not cancerous. The risk of developing breast cancer increases with age and early detection increases the chance of survival.
Symptoms could include:
- New lump or area of thickened tissue on either breast
- Change in size or shape of one or both breast
- Bloody discharge from nipples
- Lump or swelling on either armpits
- Rash around the nipple
- Change in the appearance of nipple
Men can also get breast cancer although it is very rare. In UK, about 62,000 people are diagnosed with breast cancer each year and it includes 370 men.
Symptoms of male breast cancer include:
- Nipple discharge without squeezing, often blood stained
- Inverted nipple
- Sores on the chest or nipple area
- Swelling of chest area and lymph nodes under arm
Family history plays a big factor in the likelihood of an individual in developing breast cancer. Particular genes known as BRCA1 and BRCA2 can increase risk of developing both breast and ovarian cancer.
The National Institute for Health and Care Excellence (NICE, 2013) recommends to offer mammographic surveillance annually to women aged 50 to 69 with personal history of breast cancer and are still at high risk of breast cancer and does not have TP53 mutation. And to offer MRI surveillance annually to women aged 30-49 years who has personal history of breast cancer still at risk of breast cancer.
Modalities used for breast screening includes mammography, breast ultrasonography, MRI, and clinical breast examination.
Mammography is performed for early detection of breast cancer using low dose radiation.
MRI of Breast
MRI is considered to be important diagnostic tool in detection, evaluation, treatment planning and therapy follow up of breast cancer (Mankad et al., 2011).

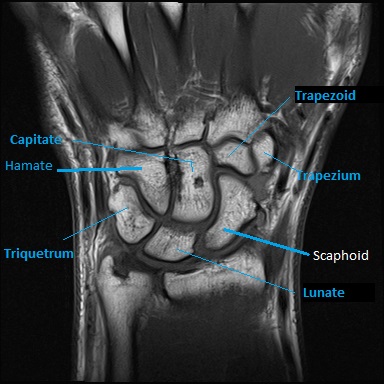
Fig 1. T2 weighted image of Breast in Axial Plane
The above image (fig 1) demonstrates anatomy of the breasts in axial plane. In order to obtain this image within optimal time-frames, parallel imaging technique (GRAPPA – Siemens) was used. This method uses multichannel coils which are coupled and works together in order to fill more lines of the K –space per given TR (Westbrook, Roth and Talbot, 2011). Each coil has its own sensitivity profile which enables to establish location of the signal depending on its amplitude and following that image is obtained or reconstructed by using algorithms (Westbrook, Roth and Talbot, 2011). The image (fig 1) was obtained in axial plane because it is considered to be more optimal comparing to sagittal plane, since reconstruction algorithms are less sensitive to movement artefacts at this instance (Thomassin-Naggara et al., 2012). T2 –weighting was used because according to the same author, it is very helpful in evaluation of cystic masses, whereas Monticciolo (2011) considers T2 weighting to be useful in detection of skin edema as sign of lymphatic obstruction. This image benefited from using T2 –weighting without application of fat saturation because according to Thomassin-Naggara et al., (2012) T2 non-fat saturated images are more informative in prediction of benign nature of cystic lesion if detected.

Fig 2. T1W
The image above (Fig 2) is T1 weighted sequence taken in axial plane. T1 is useful in detection of presence of fatty component within a lesion, which also a plays major aspect in predicting its benign nature, therefore it is done without fat saturation. T1 also allows metal markers which may have been positioned at end of biopsy to be detected (Thomassin-Nagarra et al., 2012). The same author also states that phase encoding direction for all axial plane should be right to left and for sagittal or coronal plane, the direction is anterior-posterior, to limit artefacts repeating respiratory and cardiac movement.
The patient is positioned prone with arms above head, this will limit artefacts related to phase encoding and aliasing artefact. Yeh et al.,(2014) states that positioning a patient for breast MR is technically more difficult than positioning for MR scan of different body part.
It is essential to position patient on MRI coil correctly to limit certain numbers of artefact (Thomassin-Naggara et al., 2012) as shown on table 1. Imaging efficiency and accuracy will improve in cancer detection when artefacts due to improper positioning are eliminated (Yeh et al., 2014).
| Main Artefacts | Signs | Solution |
| Movement artefact | Fuzzy lines
Propagation in the phase encoding direction Enhancement on subtracted sequences not found on native sequences |
Comfortable position for the patient
Foam wedged in the coil to wedge small breast |
| Aliasing artefact | Ghost image | Increase the FOV
Modify the phase encoding direction |
| Magnetic Susceptibility artefact | Tissue distortion
Hypersignal spots Fall in signal |
Reduce the TE |
| Non uniformity artefact of fat saturation | Occurs with a non-uniform field because of non-selection of fatty pixels | Reshim the magnet |
Table 1 shows the main artefact and solution (Thomassin-Naggara et al., 2012)
In the journal of Kriege et al., (2004) they screened 1909 women which includes 358 carriers of germ line mutation. Table 2 shows the result of the study.
| Sensitivity | Specificity | |
| Clinical Breast Examination | 17.9 | 98.1 |
| Mammogram | 33.3 | 95.0 |
| MRI | 79.5 | 89.8 |
They have compared MRI and mammography in screening women to determine whether MRI screening facilitates early diagnosis of hereditary breast cancer as MRI may improve sensitivity of screening in women with genetic predisposition to breast cancer. The study concluded that with the screening program used, MRI especially, showed more sensitivity than mammography or CBE, and can detect and breast cancer in women at early stage of risk of breast cancer, and contributed to early detection of hereditary breast cancer. However, MRI screening drawback is that its specificity is lower than mammography.
Kuhl (2004) stated that MRI seemed to be equivalent to mammography and more accurate in early familial breast cancer diagnosis. Although apart from MRI being costly, it is not eagerly accepted as screening modality for the reason of it having reported low specificity and PPV.
In the study of Warner et al., (2005) MRI, US, Mammography and CBE were performed on the same day to 236 women who were carriers of either BRCA1 or BRCA2 aged 25 to 65. 55% of women were menopausal, 30% had history of breast cancer and 9% ovarian cancer. They concluded that MRI is more sensitive for detecting breast cancers in BRCA1 and BRCA2 carriers than mammography, ultrasound and CBE alone.
Leach et al., (2005) performed a prospective cohort study participated by 649 women aged 35 to 49 with strong family history of breast or high probability mutation of BRCA1 or BRCA2 or TP3 mutation. 35 out of the 649 women screened with both mammogram and CE MRI were diagnosed with cancer, CE MRI only diagnosed 19 patients and 6 by mammography only.
| Sensitivity | Specificity | |
| Contrast Enhanced MRI | 77% | 81% |
| Mammography | 40% | 93% |
| Both modality | 94% | 77% |
Table 3 showed result of sensitivity and specificity of their study. They stated that it would benefit carriers of BRCA1 germline mutation with contrast enhanced MRI screening and combination of CE MRI and mammography will provide most effective screening examination for BRCA1, BRCA2.
This is the same with the study of Warner et al., (2008) that concluded on their study that using both MRI and mammography in screening might rule out cancerous lesions than mammography alone in women with likely inherited predisposition to breast cancer. Annual MRI and mammography screening using BI-RADS score of 4 or 5 to define positivity is accurate means in screening women with strong generic predisposition to breast cancer.
Salem et al., (2013) stated on their review article that MRI is used mostly in diagnosis and staging of breast cancer rather than in screening.
Summary
MRI showed more sensitivity than mammography in the detection of malignancy in fibroglandular or dense tissue. But as reported in the study of Leach (2005), mammography has higher specificity and combination of both mammography and CE MRI has higher sensitivity but also results to loss of specificity. Salem et al., (2013) stated that MRI might seem to be a logical choice for screening breast cancer due to its impressive ability to detect tumours not found on mammograms. However, the same authors (Salem et al.,) also said on their review article that while MRI is more sensitive than mammography, it has higher false positive rate that could result to adverse physiological effects and lead to unnecessary biopsy and screening on the women screened. There are also factors to consider such as cost effectiveness and patients with claustrophobia and devices that are not conditional or compatible with MRI.
References
Kriege, M., Brekelmans, C., Boetes, C., Besnard, P., Zonderland, H., Obdeijn, I., Manoliu, R., Kok, T., Peterse, H., Tilanus-Linthorst, M., Muller, S., Meijer, S., Oosterwijk, J., Beex, L., Tollenar, R., Koning, H., Rutgers, E. and Klijn, J. (2004) Efficacy of Mri and Mammography For Breast-cancer Screening in Women with a Familial Or Genetic Predisposition. The New England Hournal of Medicine [online]. 351 (5), pp. 427-437. [Accessed 30 March 2019].
Kuhl, C. (2004) Screening of Women with Hereditary Risk of Breast Cancer. Clinical Breast Cancer [online]. 5 (4), pp. 269-271. [Accessed 28 March 2019].
Leach, M.O., Boggis, C.R., Dixon, A.K., Easton, D.F., Eeles, R.A., Evans, D.G., Gilbert, F.J., Griebsch, I., Hoff, R.J., Kessar, P., Lakhani, S.R., Moss, S.M., Nerurkar, A., Padhani, A.R., Pointon, L.J., Thompson, D., Warren, R.M. and , (2005) Screening with Magnetic Resonance Imaging and Mammography of a Uk Population at High Familial Risk of Breast Ca: A Prospective Multicentre Cohort Study (Maribs). The Lancet [online]. 365 (9473), pp. 1769-1778. [Accessed 28 March 2019].
Mankad, K., Hoey, E., Lakkaraju, A., and Bhuskute, N., 2011. MRI of the whole body. An illustrated guide to common pathologies. London: Hodder Arnold.
Monticciolo, D. L., 2011. Magnetic Resonance Imaging of the breast for cancer diagnosis and staging. Seminars in Ultrasound CT and MRI [online]. 32 (4), pp. 319-330. [Accesses 28 March 2019].
Salem, D., Kamal, R., Mansour, S., Salah, L. and Wessam, R. (2013) Breast Imaging in the Young: The Role of Magnetic Resonance Imaging in Breast Cancer Screening, Diagnosis and Follow Up. J Thorac Dis [online]. 5 (1), pp. 9-18. [Accessed 28 March 2019].
The National Institute for Health and Care Excellence, NICE (2013) Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer. Available from: https://www.nice.org.uk/guidance/cg164/chapter/Recommendations#surveillance-and-strategies-for-early-detection-of-breast-cancer [Accessed 29 March 2019].
Thomassin-naggara, I., Trop, I., Lalonde, L., David, J., Peloquin, L. and Chopier, J. (2012) Tips and Techniques in Breast Mri. Diagnostic and Interventional Imaging [online]. 93, pp. 828-839. [Accessed 28 March 2019].
Warner, E., Messersmith, H., Causer, P., Eisen, A., Shumak, R. and Plewes, D. (2008) Systematic Review: Using Magnetic Resonance Imaging to Screen Women at High Risk For Breast Cancer. Annals of Internal Medicine [online]. 148 (9), pp. 671-679. [Accessed 28 March 2019].
Warner, E., Plewes, D.B., Hill, K.A., Causer, P.A., Zubovits, J.T., Cutrara, M.R., Yaffe, M.J., Messner, S.J., Meschino, W.S., Piron, C.A. and Narod, S.A. (2005) Surveillance of Brca1 and Brca2 Mutation Carriers with Magnetic Resonance Imaging, Ultrasound, Mammography, and Clinical Breast Examination. Evidence Based Obstetrics and Gynecology [online]. 7 (2), pp. 100-102. [Accessed 29 March 2019].
Westbrook, C., Roth, C. K. and Talbot, J., 2011. MRI in practice.4th ed. Chichester: Wiley-Blackwell.
Yeh, E., Georgian-smith, D., Raza, S., Bussolari, L., Pawlisz-hoff, J. and Birdwell, R. (2014) Positioning in Breast Mr Imaging to Optimize Image Quality. Radiographics 2014 [online] 34 (1). Available from: https://doi.org/10.1148/rg.341125193 [Accessed 30 March 2019].
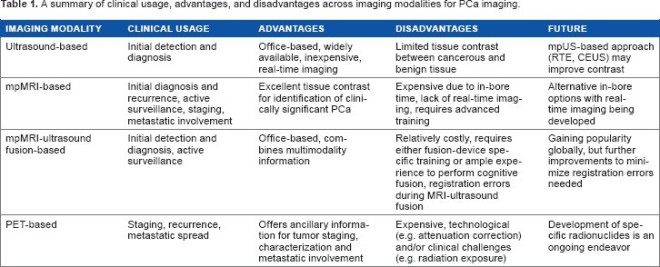
 Fig 3B
Fig 3B